|
|
Calculations with Moles
|
The chart below is the heart of the moles chart. This details the calculations needed to convert various amounts to and from moles.
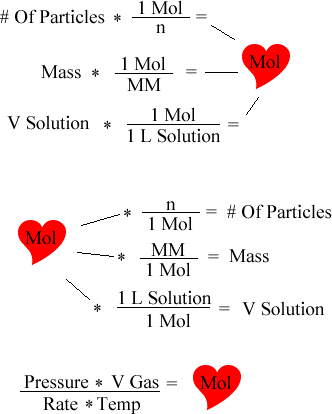
Calculating Moles and numbers of particles.
We shall start the demonstration of how to get moles from a given number of particles. If we are given the problem to find how many moles are in 5.0 X 10^23 number of particles, we simply do the following. We divide the number of particles we are given by Avogadro's Number. We can reverse the conversion by merely multiplying moles and Avogadro's number. Below is an example.
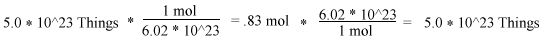
If we are given the problem to find how many moles are in 56.0 grams of Oxygen, we do the following. We divide the mass we are given by Molar Mass of the element or compound. We can reverse the conversion by multiplying moles and the molar mass. Below is an example.

If we are given the problem to find how many moles are in 2 liters of a 5M solution, we do the following. We multiply the liters of the solution by the Molarity rate of 1 mol over 1 L solution. We can reverse the conversion by multiplying by the opposite of the molarity rate, 1 L solution over 1 mol. Below is an example.

If we are given the problem to find how many moles are in 2 liters a gas at standard temperature and pressure. To find out we do the following. We multiply the liters of the gas by the Pressure divided by the constant gas rate times temperature. We can reverse the equation by solving it algebrically for V.
|
|