





|
|
What are the definitions of a mole?
|
There are several definitions of a mole. Here I shall put the ones that relate to chemistry.
Molar Mass The mass of one mole of any element. Relating to the Latin moles meaning "mass".
Molarity Concentration of moles per single liter of solution (rate from liters of solution to mols.); from the Latin moles meaning "mass".
Molar Volume of a gas The volume of a mole of an element or compound in gas form at a given temperature and a given pressure.
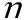 is used to represent a mole in algebric equations. is used to represent a mole in algebric equations.
mol is the abbrivated term for a mole. It is used as a label in chemical equations.
The mol abbrivation is also used in the rate of molarity.
|
|



