

Fuel Cells
A fuel cell converts the chemicals hydrogen and oxygen into water, and in the process it produces electricity. Someday soon, we may using the new energy-saving technology to generate electrical power for our homes and cars.
There are several different types of fuel cells, each using a different chemistry. Fuel cells are usually classified by the type of electrolyte they use. Some types of fuel cells work well for use in stationary power generation plants. Others may be useful for small portable applications or for powering cars.
The proton exchange membrane fuel cell (PEMFC) is one of the most promising technologies. This is the type of fuel cell that will end up powering cars, buses and maybe even your house.


A fuel-cell stack that could power an automobile
To see an animation of how a fuel cell works, go to this link: http://auto.howstuffworks.com/fuel-cell2.htm
More information about fuel cells is available on these sites:
http://pcworld.about.com/magazine/1904p076id41670.htm
http://pcworld.about.com/news/Apr032003id110120.htm
Bacterial Batteries
In the quest for batteries that are still more environmentally friendly, some researchers are even turning to the mud of the sea floor. Microbiologists at the University of Massachusetts at Amherst have tapped into the energy produced by a group of bacteria commonly found in ocean floor sediment to produce electricity.
These bacteria, of the family Geobacteraceae, break down the organic matter on the ocean floor to get the energy they need to live, and as they do, they release a stream of electrons that could be channeled into producing electricity. An anode buried in sea mud was connected with a copper wire to a cathode in the seawater, and as the bacteria stripped electrons from organic compounds in the mud, the electrons flowed from the anode to the cathode, producing an electric current. (As an added bonus, these bacteria have shown the ability to degrade toxic organic pollutants, such as benzene, by converting them into carbon dioxide and other benign wastes.) To date, the researchers envision the minimal current produced being useful for such applications as powering remote sensor stations, thus avoiding the necessity of long trips to change batteries. The study is still in its early stages, and thus far, power outputs are minimal, but it shows promise.
Carbon Nanotubes
Otto Zhou is director of the North Carolina Center for Nanoscale Materials at the University of North Carolina at Chapel Hill. Zhou and his team are looking into the submicroscopic world of nanotubes to find new ways to store energy in batteries.
Nanotubes with an affinity for lithium ions may be the power storage solution of the future.
image credit: J. Fischer, Matt Ray/EHP
Lithium ion batteries typically use graphite or a carbon material as one of the electrodes. The electrode materials limits the amount of energy that can be stored. Usually a graphite electrode stores one charged lithium ion for every six carbon atoms, but it was found that with nanotubes, one charged lithium ion can be stored for every three carbons.
How Power Paper Will Work


Power Paper has developed an
ultra-thin battery that can generate 1.5 volts of power.
Power Paper, an Israel-based company, has recognized the need for a thinner power source that will not only power electronic devices, but also fit into disposable devices like games, greeting cards, smart cards, luggage tags and some medical devices. And as Power Paper is looking for companies to license its paper-thin battery technology to, it is already developing disposable products using the technology that will debut in the second half of 2001.
With the development of computerized clothing, wearable computers and disposable cell phones, this ultra-thin battery technology is likely to have widespread applications. Power Paper will work exactly like a traditional battery, but it will be nearly as thin as a piece of paper. A Power Paper cell can generate 1.5 volts of electricity, which is about the same output as a watch or calculator battery. A Power Paper cell will be 0.5 millimeters thick, and several cells can be used in combination to provide more power.
Microengines: The batteries of the future
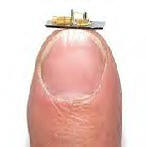
University of Birmingham engineers have developed tiny engines only a few millimetres wide, that could soon replace a standard battery.
Lead by Dr. Kyle Jiang, investigators at the University's School of Engineering are the first to manufacture these engines in a durable, heat resistant material such as a ceramic or silicon carbide. As a result, these micro-engines will have over 300 times more energy than an ordinary battery and are much lighter and smaller.
These new power-supplying machines could be used to charge mobile phones and laptop computers in a matter of seconds and could also be used for a range of other applications such as micro-factories, tiny "labs-on-a-chip" that will be able to make drugs, chemicals or small mechanical components.