|
Baghdad Battery
 Approximately 2000 years
old
|
 |
|
In 1937, William Koenig became the
director of the Museum in Baghdad. He thought that one of
the artifacts in the museum could have been used as a battery.
The yellow clay pot, dated around 200 BC, was the object Koenig
was questioning. The pot was from the Parthian era,
however, they were not known for any cultural or scientific
achievements. They were warriors. If this really is
an ancient battery, it was most likely used for gilding -
putting sheet gold onto silver statuettes or vases.
Another possibility may have been to use the low voltage,
approximately 1.5 V if pineapple juice had been used, for health
reasons.
TOP |
|
|
|
Voltaic Pile


This model of the first voltaic pile is
at the National Museum of American History in Washington, D. C. |
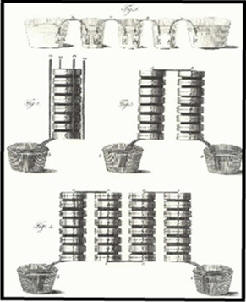
Original drawings of his Piles by Volta: the "chain of
cups" apparatus (upper part), and the "columnar apparatus" (middle and
lower parts) |
|
|
The first battery was created by Alessandro
Volta in 1800. To create his battery, he made a stack by
alternating layers of zinc, blotting paper soaked in salt water,
and silver. This
device produced a small but steady electrical current.
Contact between the two metals creates a difference in potential
(or pressure, or "voltage"), which in a closed circuit produces
electric current. Voltaic piles mark the origin of modern
batteries.
TOP
|
|
|
Leyden Jars


This was the first-generation capacitor
for storage of electric charge.
|
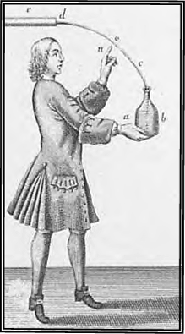

Early illustration of Leyden Jars
From Nollet's "Lettres
sur L'Electricite, 1751 |
|
|
Leyden Jars are capacitors that store
static charges. The glass jar serves as a dielectric medium
and contact plates were metal foils wrapped, inside and out, around
the cylindrical surfaces of the jar. A conducting electrolyte
solution, originally water, later other liquids, was inside the jar.
The metal electrodes were placed in the liquid and the device was
charged by joining two wires form the inside electrode and the
outside foil to an electrostatic machine. Later, the Leyden
Jar capacitor was connected to the electrodes of a Volta's Pile or
battery for charging.
TOP |
|
|
|
|
Dry Cell


The Zinc Carbon Dry Cell or Leclanché batteries
|
Morse Telegraph
After many years of experimentation, the “dry cell” was invented in the
1860s for use with the telegraph.

National Museum of American History
in Washington, D. C.
Pulses of electricity caused the two vertical electromagnets (on the
right) to pull against an iron bar attached to the horizontal brass
lever arm.
|
|
|
In 1866, George Leclanché developed
what would be the forerunner of the world's first widely used
battery the zinc carbon cell. The positive electrode consisted of
crushed manganese dioxide with a little carbon mixed in. The
negative pole was a zinc rod. The cathode was packed into the pot,
and a carbon rod was inserted to act as a currency collector. The
anode or zinc rod and the pot were then immersed in an ammonium
chloride solution. The liquid acted as the electrolyte, readily
seeping through the porous cup and making contact with the cathode
material.
TOP |
|
| |
|
|
 |
|
|
|
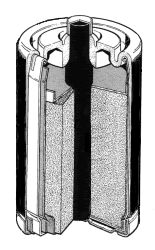
In the 1880s, Carl Gassner invented
the first commercially successful dry cell battery, a zinc-carbon cell.
Zinc was used as the outer container and also served as the negative
electrode. The positive electrode, a carbon rod, was immersed in a
manganese dioxide/carbon black mixture. It was separated from the zinc
container by a folded paper sack, soaked in a solution of ammonium
chloride which acted as the electrolyte. The electrolyte was absorbed in
a porous material and the cell was sealed across the top. During use the
zinc casing of the battery was gradually consumed by the chemical
reaction. A bitumen seal prevented evaporation of water from the
electrolyte and the ingression of oxygen. This cell was easy to handle
and portable. It became the prototype for the dry battery industry.
Patent information for Gassner's zinc-carbon
cell is available here:
No. 373,064
TOP |
|
|
|
Edison's Battery |
 |
|
|
After 50,000 experiments on the storage battery, in 1910 he produced
his successful nickel-iron-alkaline storage battery. The
Edison alkaline battery proved useful for lighting railway cars and
signals, maritime buoys, and miners lamps. |
|
TOP |
|
|
|
| Nickel Cadmium Batteries
 |
 |
|
|
In 1899, Waldmar Jungner
invented the first nickel-cadmium rechargeable battery. In 1947, Neumann succeeded
in completely sealing the cell. These advances led to the modern sealed
nickel-cadmium battery in use today.
In August, 1959, broad flexibility of nickel-cadmium battery designs is demonstrated
by two newly developed multiple cell Burgess batteries.
The six-volt battery shown above contains five flat nickel-cadmium cells
stacked in a column and connected in series. Eight individual cells form
the 9.6-volt battery to the right of the 6-volt battery.
By combining cells in this manner, using a unique conductive silver wax
inter-cell connection, Burgess engineers can design and mass produce a
virtually unlimited variety of rechargeable nickel-cadmium batteries to
meet the special requirements of industrial designers and electrical
engineers.
TOP
|
|
|
|
|
|
Tiny Dry Battery
|
 |
|
| January 28, 1946
Tiny dry battery that powered the “handie-talkie” used by Army
Signal Corps has now been developed into the commercial model
shown in the picture, used principally in hearing aids.
Chemical reaction of zinc and mercuric oxide operates the cell;
conventional cells use zinc and carbon.
TOP
|
|
|
|

|
World's Smallest Battery |
|
|
December 20, 1965
Close-up of a new high-energy mercury battery, the smallest of
its kind in the world and intended for use in “all-in-the-ear”
hearing aids.
Weighing less than 1/4 of an ounce, and 1/8” in diameter, it is half
the size of an aspirin tablet. The tiny 1.35 volt Mallory battery
provides the highest watt-hours of any commercially available
battery of its size and kind.
Shown here in a comparison of size with a pencil, it is rated at 16
milliampere hours.
TOP
|
|
|
To learn more about the
history of batteries, visit these sites:
History of Batteries - Energizer.com
http://www.energizer.com/learning/historyofbatteries.asp
Timeline of Battery
History
http://inventors.about.com/library/inventors/blbattery.htm
|
|
TOP
|
|
|
|
|
|
|
|
|



