#5
Immune deviation Following Pulse Cyclophosphamide/MethylPrednisolone Treatment of Multiple Sclerosis: Increased InterLeukin-4 Production & Associated Eosinophilia
Smith DR; Balashov KE; Hafler DA; Khoury SJ; Weiner HL
Ann Neurol 1997 Sep, 42:3, 313-8
Harvard Medical School, Center for Neurologic Diseases, Brigham and Women's Hospital, Boston, MA 02115, USA
UI# 97450811
Abstract
Multiple Sclerosis (MS) is postulated to be a Th1-type Cell-mediated AutoImmune Disease.
Thus therapies that decrease T-Cell Interferon-gamma (IFN-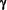 ) production or increase InterLeukin-4 (IL-4) production would be expected to have an ameliorating effect on MS. ) production or increase InterLeukin-4 (IL-4) production would be expected to have an ameliorating effect on MS.
Some Progressive MS patients receiving pulse Cyclophosphamide therapy developed peripheral blood Eosinophilia. We investigated whether Cyclophosphamide treated patients had Immune Deviation toward Th2 responses.
We measured Cytokine production in patients receiving either monthly IntraVenous MethylPrednisolone (MP), IntraVenous Cyclophosphamide plus MethylPrednisolone (CY/MP), Methotrexate, IFN-ß-1b, in untreated MS patients, and in healthy controls.
Minimal IL-4 was secreted in untreated patients (129 +/- 62 pg/ml), Methotrexate treated patients (99 +/- 79 pg/ml), and healthy controls (50 +/- 13 pg/ml). A marked increase in IL-4 was observed in CY/MP patients (1,503 +/- 291 pg/ml).
Patients treated with MP (418 +/- 160 pg/ml) or IFN-ß-1b (425 +/- 167 pg/ml) showed small increases. Eosinophilia in CY/MP-treated patients (6.0 +/- 0.7%) correlated with increased IL-4. IL-10 production was also increased in CY/MP-treated patients.
Both CY/MP- and MP-treated groups had decreased production of IFN-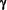 compared with untreated MS. compared with untreated MS.
These findings demonstrate pronounced Immune Deviation favoring Th2-type responses after pulse Cyclophosphamide therapy.
#6
Side-Effects of IV Cyclophosphamide Pulse Therapy
Martin F; Lauwerys B; Lefèbvre C; Devogelaer JP; Houssiau FA
Lupus, 1997, 6:3, 254-7
Saint-Luc Univ Hospital, Louvain Medical School, Rheumatology Dept, Bruxelles, Belgium
UI# 97258258
Abstract
We reviewed the side-effects of IntraVenous (I.V.) Cyclophosphamide (CPM) pulse therapy in a group of 75 patients suffering from various AutoImmune disorders (mostly Systemic Lupus Erythematosus and Vasculitis) who received a total of 451 I.V.
CPM pulses, given on a monthly basis (mean +/- s.d. CPM dose per pulse: 764 +/- 217 mg; mean +/- s.d. follow-up period: 26.7 +/- 22.1 mon).
Infection was the most common side-effect (30 episodes in 21 patients; 28% of the patients) but rarely required in-patient treatment (8 episodes in 7 patients; 9% of the patients).
No relationship could be found between the occurrence of infection and the dose of CPM or of GlucoCorticoids. Other side-effects were rare. Only one patient suffered from Neutropenia.
Haemorrhagic Cystitis was never observed nor did premature Ovarian failure in the 25 female patients at risk.
Four patients developed Neoplasia and three died suddenly a few days after receiving a CPM pulse but the causal relationship between CPM therapy and these poor outcomes is speculative.
Taken together, our data confirm in a large group of patients that I.V. CPM pulse therapy is relatively safe. In particular, the rate of severe infection requiring in-patient treatment is rare (1.8% of 451 pulses.).
#7
Multiple Spontaneous IntraCerebral Hemorrhages in Progressive Systemic Sclerosis
Andonopoulos AP; Maraziotis T; Rigas G; Yarmenitis S; Papapetropoulos T
Rev Rhum Engl Ed 1998 Jun, 65:6, 437-40
Patras Regional Univ Hospital, Division of Rheumatology, Univ of Patras School of Medicine, Greece
UI# 98334993
Abstract
A 64-year-old woman with a two-year history of diffuse Scleroderma responsible for severe Interstitial Lung Disease was admitted for recurrent loss of consciousness.
Her treatment at the time consisted of a CorticoSteroid and monthly Cyclophosphamide pulses.
Computed Tomography and Magnetic Resonance Imaging of the Brain revealed two Hemorrhagic lesions in the Left Frontal and Temporal Lobes, respectively.
Amyloidosis and/or Vasculitis may have contributed to these Lesions.
#8
Stabilization Of Rapidly Worsening Multiple Sclerosis For 36 Months In Patients Treated With Interferon-ß Plus Cyclophosphamide Followed By Interferon-ß
Patti F, Reggio E, Palermo F, Fiorilla T, Politi G, Nicoletti A, Reggio A
J Neurol 2004 Dec;251(12):1502-6
Department of Neurological Sciences, Via Santa Sofia 78, 95123, Catania, Italy
PMID# 15645351
Abstract
Cyclophosphamide (CTX) is an Alkylating agent related to Nitrogen Mustards, whose AntiInflammatory and ImmunoSuppressive effects have been utilized to treat selected cases, of Multiple Sclerosis with a Progressive and worsening course.
To halt the progression of disease, in patients refractory to disease modifying drugs, CTX has been given, and several open-label studies have recently shown clinical benefits.
In a previous study we demonstrated the effectiveness of a combination of IV monthly pulses of CTX and Interferon-beta (IFN-ß), in 10 patients with "rapidly transitional" form of Multiple Sclerosis. Characterized by severe and frequent attacks and rapid progression of disability.
The present study reports the clinical and MRI follow-up, compared to the pre-treatment period, 36 months after the discontinuation of CTX.
Showing the maintenance of the results obtained in:
- Relapse Rate (p < 0.001)
- EDSS (p < 0.001)
- T2 MRI total lesion load (p < 0.001)
- T2 lesions number (p < 0.001)
These encouraging findings and the absence of significant recorded side effects, affirm that the association of CTX plus Interferon-ß is amenable, safe and can be recommended in rapidly worsening MS patients.
#9
Multiple Sclerosis: Long-Term Remission After A High Dose Of Cyclophosphamide
Bittencourt PR, Gomes-da-Silva MM
Acta Neurol Scand 2005 Mar;111(3):195-8
Unidade de Neurologia Clinica, Curitiba, Brazil
PMID# 15691289
Abstract
The objective of this case report is to document the possibility that ImmunoAblative doses of Cyclophosphamide may provide a long-term remission of Multiple Sclerosis (MS).
We report the case of a 48-year-old woman with Definite MS diagnosed in 1994 who has been in complete remission since a dose of 3800 mg of Cyclophosphamide was accidentally given intravenously in early 1997.
For 7 years there have been no signs of disease activity on history, physical examination, or on high-quality Magnetic Resonance Imaging (MRI) with appropriate contrast-enhancement methodology.
This case includes information on the possibility that less aggressive ChemoTherapy than that used with Stem Cell Transplantation may be effective in the long-term control of MS.
#10
Cyclophosphamide Is Effective In Stabilizing Rapidly Deteriorating Secondary/Progressive Multiple Sclerosis
Perini P, Gallo P
J Neurol 2003 Jul;250(7):834-8
Multiple Sclerosis Center, First Neurology Clinic, Department of Neurological & Psychiatrical Sciences, University of Padova, Via Giustiniani 5, 35128 Padova, Italy
PMID# 12883926
Abstract
The safety and efficacy of pulse Cyclophosphamide (CTX) therapy was investigated in patients with very active Secondary/Progressive Multiple Sclerosis, characterized by frequent relapses and rapid disability progression.
For this purpose the clinical and MRI effects were assessed.
Sixteen patients, 11 female and 5 male, were experiencing rapidly deteriorating disease, characterized by frequent and severe relapses as well as rapid progression (defined by an increase of more than 1 EDSS point in a period of 1 year).
Mean relapse rate in the two years preceding CTX therapy was 3.0 +/-1.4. Mean EDSS was 4.0+/-1.4 one year before therapy and 5.6+/-1.0 at study entry.
Treatment consisted in administration of high dose intravenous CTX every four weeks for one year and then every eight weeks for an additional twelve months.
CTX dose was tailored to the patient's white blood cell response, and ranged from 800 to 1,200 mg/m(2) body surface. MRI was performed before therapy and then at 12 (Y1) and 24 (Y2) months.
Eight patients with similar clinical features constituted a control group. CTX therapy was safe and well tolerated, and no severe side effects were observed.
The EDSS decreased to 4.3+/-1.6 at Y1 (Y0 vs.Y1: p< 0.001) and to 4.1+/-1.6 at Y2 (Y0 vs.Y2: p< 0.001). Only four patients experienced relapses during the first year of therapy, while no relapses were observed during the second year of therapy.
The mean relapse rate during therapy was 0.25 +/-0.45 (p< 0.0001).No increase in T2 lesion load was observed over the two years.
A significant clinical and MRI deterioration was observed in the control group. Therapy with pulse CTX was able to stop disease activity and progression in patients with rapidly evolving Secondary/Progressive MS.
#11
Treatment Of Progressive Forms Of Multiple Sclerosis By Cyclophosphamide: A Cohort Study Of 490 Patients
Zephir H, de Seze J, Duhamel A, Debouverie M, Hautecoeur P, Lebrun C, Malikova I, Pelletier J, Senechal O, Vermersch P
J Neurol Sci 2004 Mar 15;218(1-2):73-7
Hopital R. Salengro, CHRU of Lille, Department of Neurology, 59037, Lille, cedex, France
PMID# 14759636
Abstract
There are no generally effective disease-modifying drugs for Progressive forms of Multiple Sclerosis (MS). Some MS centres use Cyclophosphamide (CYC) in Secondary/Progressive (SP) forms of MS, especially after Interferon-beta-1b (INF-beta-1b) treatment failure.
Moreover, there are currently no approved drugs for Primary/Progressive (PP MS). Using the collected data of patients with Progressive MS, we studied clinical patterns that predicted a good response to CYC treatment.
Secondly, we compared the therapeutic response of SPMS and PPMS patients to the treatment. Data from 490 MS patients were collected.
All patients presented an SP (n = 362) or PP (n = 128) form of the disease and 476 had been treated for at least one year with a monthly pulse of CYC associated with MethylPrednisolone (MP).
CYC treatment was justified because of at least a 1-point worsening on the Expanded Disability Status Scale (EDSS) during the previous year.
The EDSS score was assessed at baseline and after 6 months (M6) and 12 months (M12) of treatment.
After 12 months of CYC treatment, 78.6% of SPMS and 73.5% of PPMS patients had stabilized or had an improved EDSS score. Response to CYC was not significantly different in the two Progressive forms of MS.
Twenty-two patients presented noticeable drug side effects, one of whom withdrew from the treatment due to intolerance.
Patients with an improved EDSS at M12 had a shorter mean progressive time course (5.1 years) than patients who stabilized or worsened (7.1 years) (p = 0.02). We also observed that poor responders at M6 were also poor responders at M12 (p < 0.001).
This large cohort study showed that CYC treatment was well tolerated and suggested that a better response occurred in cases with a short progressive time course.
We did not find any difference in treatment response between the two Progressive forms of MS.
To date, no treatment is approved for PPMS and we therefore propose a trial to test the use of CYC treatment early in the course of the disease in PPMS patients with disability progression.
#12
Treatment Of Progressive Multiple Sclerosis With Monthly Pulsed Cyclophosphamide-MethylPrednisolone: Predictive Factors Of Treatment Response
Delmont E, Chanalet S, Bourg V, Soriani MH, Chatel M, Lebrun C
Rev Neurol (Paris) 2004 Jul;160(6-7):659-65
Service de Neurologie, CHU de Nice
PMID# 15247854
Abstract
Introduction
Cyclophospamide is used in the treatment of Progressive Multiple Sclerosis. We were looking for predictive indicators of treatment response.
Material And Methods
Forty-seven patients with Secondary Progressive Multiple Sclerosis and seven others with Primary/Progressive received monthly infusions of Cyclophosphamide (750mg/m2) and MethylPrednisolone (500mg).
During the year before Cyclophosphamide the EDSS had worsened one point in all patients with or without surimposed relapses. Evaluation was based on EDSS change at 6, 12, 24 months and 5 years.
Results
Among Secondary/Progressive patients, 91 per 100 (43/47) were stable or improved at 12 months, 65 per 100 (26/40) at 24 months and 22 per 100 (5/23) at 5 years.
Annual relapse rate decreased from 0.81 before treatment to 0.48 during treatment and 0.12 after treatment (p<0.001).
At 24 months, efficacy was correlated to a progressive phase lasting less than 5 years (p< 0.01) and to a rapid increase of EDSS of at least 2 points the year before treatment (p< 0.05).
There were no influences of age, EDSS and surimposed relapses at the beginning of treatment, and other immunoactive drugs administrated before Cyclophosphamide.
There was no significant difference in quality of response to treatment between patients with primary progressive and Secondary/Progressive Multiple Sclerosis.
Conclusion
Cyclophosphamide appears to be more efficient in early stage of Progressive Multiple Sclerosis independently of age, relapses or Neurological disability scale.
|